Pipeline Overview
G1 is advancing its lead clinical compound trilaciclib, a first-in-class therapy designed to improve outcomes for patients with certain cancers, in clinical trials assessing combinations with cytotoxic therapies including antibody-drug conjugates (ADCs) and/or immunotherapy in areas of high unmet need including triple-negative breast cancer and extensive stage small cell lung cancer.
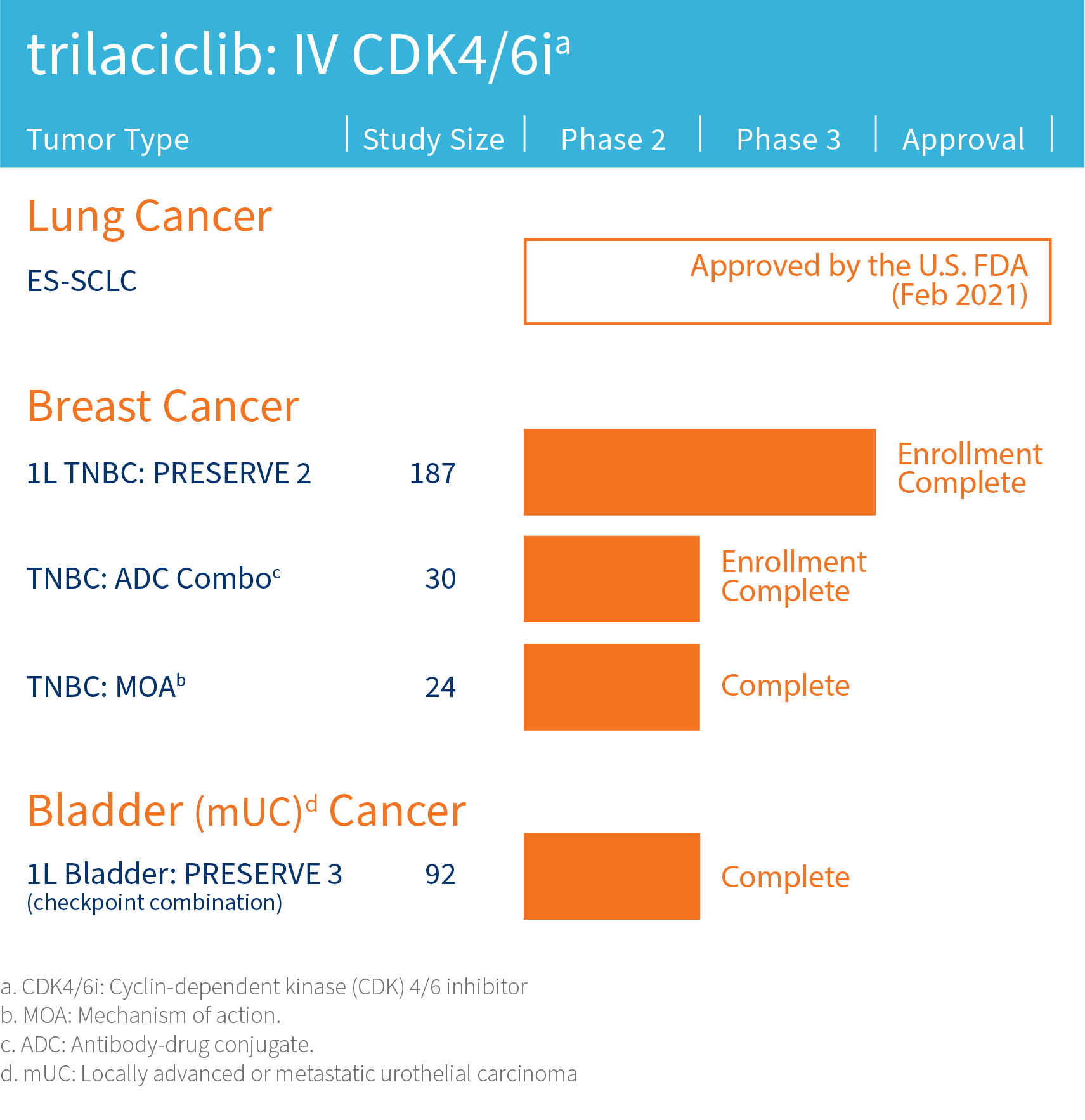
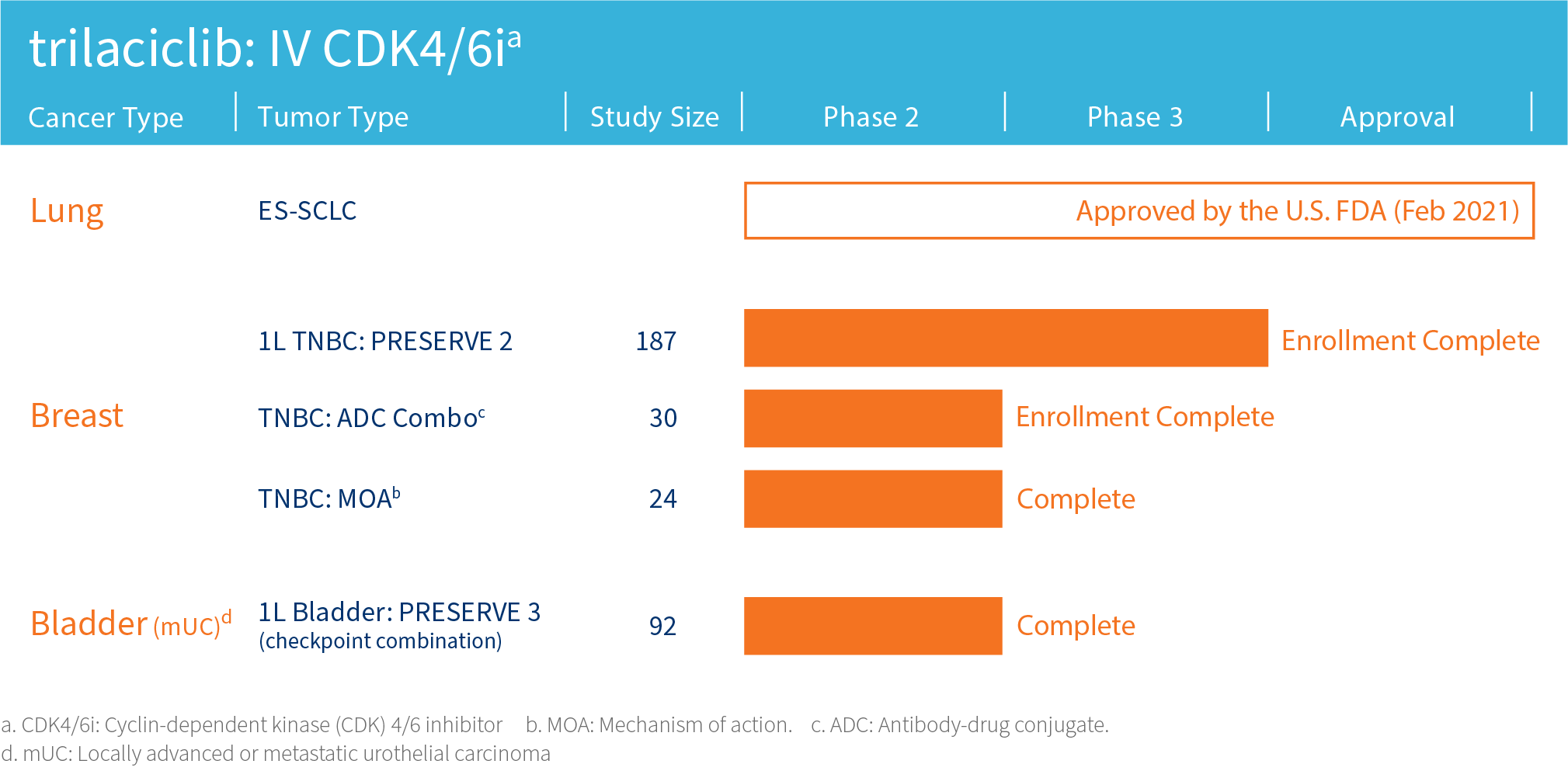
The safety and efficacy of investigational agents or an investigational use of an approved product have not been established or approved by the FDA or other regulatory authorities.